Diagnostic Medical Device Classification . This guidance, relating to the application of regulation (eu) 2017/746 on in vitro diagnostic medical devices (ivdr) addresses the. This scheme assigns diagnostic medical devices to one of four classes (a through d) based on ascending risk to patients if the device malfunctions, using seven. Any active medical device, whether used alone or in combination with other medical devices, to. The imdrf guidance documents essential principles of safety and performance of medical devices and ivd medical devices and. The fda classifies medical devices, including ivd products, into class i, ii, or iii according to the level of regulatory control that is necessary to. This document provides guidance to assist product owners in risk classification of in vitro diagnostic (ivd) medical devices using the. Active device intended for diagnosis: Medical device (md) is defined in the first schedule of the health products act (hpa) and products that meet this definition are regulated.
from www.pacificbridgemedical.com
This guidance, relating to the application of regulation (eu) 2017/746 on in vitro diagnostic medical devices (ivdr) addresses the. Medical device (md) is defined in the first schedule of the health products act (hpa) and products that meet this definition are regulated. This scheme assigns diagnostic medical devices to one of four classes (a through d) based on ascending risk to patients if the device malfunctions, using seven. Any active medical device, whether used alone or in combination with other medical devices, to. The imdrf guidance documents essential principles of safety and performance of medical devices and ivd medical devices and. Active device intended for diagnosis: The fda classifies medical devices, including ivd products, into class i, ii, or iii according to the level of regulatory control that is necessary to. This document provides guidance to assist product owners in risk classification of in vitro diagnostic (ivd) medical devices using the.
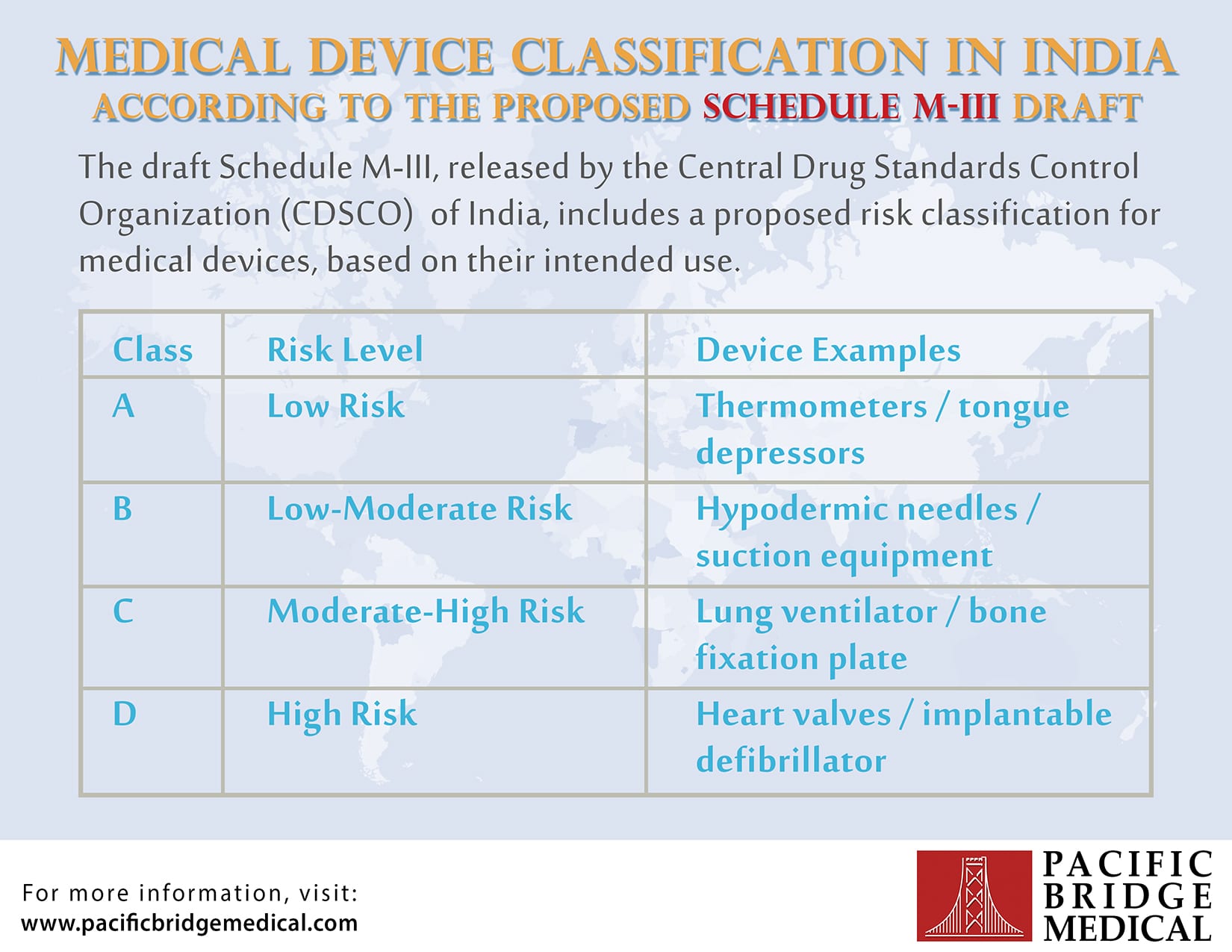
Device Classification in India Infographic
Diagnostic Medical Device Classification Medical device (md) is defined in the first schedule of the health products act (hpa) and products that meet this definition are regulated. Active device intended for diagnosis: This scheme assigns diagnostic medical devices to one of four classes (a through d) based on ascending risk to patients if the device malfunctions, using seven. This document provides guidance to assist product owners in risk classification of in vitro diagnostic (ivd) medical devices using the. The imdrf guidance documents essential principles of safety and performance of medical devices and ivd medical devices and. The fda classifies medical devices, including ivd products, into class i, ii, or iii according to the level of regulatory control that is necessary to. Medical device (md) is defined in the first schedule of the health products act (hpa) and products that meet this definition are regulated. This guidance, relating to the application of regulation (eu) 2017/746 on in vitro diagnostic medical devices (ivdr) addresses the. Any active medical device, whether used alone or in combination with other medical devices, to.
From meddev-info.blogspot.com
Medical Device Regulation Basics US FDA Medical Device Classification Diagnostic Medical Device Classification The fda classifies medical devices, including ivd products, into class i, ii, or iii according to the level of regulatory control that is necessary to. Any active medical device, whether used alone or in combination with other medical devices, to. Active device intended for diagnosis: The imdrf guidance documents essential principles of safety and performance of medical devices and ivd. Diagnostic Medical Device Classification.
From angelanjohnson.com
Medical Devices Angela N Johnson Diagnostic Medical Device Classification Active device intended for diagnosis: Medical device (md) is defined in the first schedule of the health products act (hpa) and products that meet this definition are regulated. The imdrf guidance documents essential principles of safety and performance of medical devices and ivd medical devices and. This guidance, relating to the application of regulation (eu) 2017/746 on in vitro diagnostic. Diagnostic Medical Device Classification.
From vyomusconsulting.com
MedicalDevice & IVD Registration Process Overview Diagnostic Medical Device Classification This scheme assigns diagnostic medical devices to one of four classes (a through d) based on ascending risk to patients if the device malfunctions, using seven. This guidance, relating to the application of regulation (eu) 2017/746 on in vitro diagnostic medical devices (ivdr) addresses the. The fda classifies medical devices, including ivd products, into class i, ii, or iii according. Diagnostic Medical Device Classification.
From www.greenlight.guru
Understanding the FDA Medical Device Classification System Diagnostic Medical Device Classification The fda classifies medical devices, including ivd products, into class i, ii, or iii according to the level of regulatory control that is necessary to. Medical device (md) is defined in the first schedule of the health products act (hpa) and products that meet this definition are regulated. This guidance, relating to the application of regulation (eu) 2017/746 on in. Diagnostic Medical Device Classification.
From www.greenlight.guru
Medical Device Classification Guide How To Determine Your Device Class Diagnostic Medical Device Classification Medical device (md) is defined in the first schedule of the health products act (hpa) and products that meet this definition are regulated. The imdrf guidance documents essential principles of safety and performance of medical devices and ivd medical devices and. This guidance, relating to the application of regulation (eu) 2017/746 on in vitro diagnostic medical devices (ivdr) addresses the.. Diagnostic Medical Device Classification.
From www.qualio.com
Medical device classification guide Diagnostic Medical Device Classification This document provides guidance to assist product owners in risk classification of in vitro diagnostic (ivd) medical devices using the. Medical device (md) is defined in the first schedule of the health products act (hpa) and products that meet this definition are regulated. This guidance, relating to the application of regulation (eu) 2017/746 on in vitro diagnostic medical devices (ivdr). Diagnostic Medical Device Classification.
From synectic.net
Medical Device FDA Regulations Infographic Synectic Diagnostic Medical Device Classification Active device intended for diagnosis: This scheme assigns diagnostic medical devices to one of four classes (a through d) based on ascending risk to patients if the device malfunctions, using seven. Any active medical device, whether used alone or in combination with other medical devices, to. The imdrf guidance documents essential principles of safety and performance of medical devices and. Diagnostic Medical Device Classification.
From www.arenasolutions.com
How to Classify Your Medical Device Under the EU MDR and IVDR Arena Diagnostic Medical Device Classification This guidance, relating to the application of regulation (eu) 2017/746 on in vitro diagnostic medical devices (ivdr) addresses the. This document provides guidance to assist product owners in risk classification of in vitro diagnostic (ivd) medical devices using the. Medical device (md) is defined in the first schedule of the health products act (hpa) and products that meet this definition. Diagnostic Medical Device Classification.
From emmainternational.com
Classifying Medical Devices under EU MDR Diagnostic Medical Device Classification This scheme assigns diagnostic medical devices to one of four classes (a through d) based on ascending risk to patients if the device malfunctions, using seven. Any active medical device, whether used alone or in combination with other medical devices, to. This document provides guidance to assist product owners in risk classification of in vitro diagnostic (ivd) medical devices using. Diagnostic Medical Device Classification.
From chinameddevice.com
CFDA New Medical Device Classification Catalog China Med Device Diagnostic Medical Device Classification This scheme assigns diagnostic medical devices to one of four classes (a through d) based on ascending risk to patients if the device malfunctions, using seven. Medical device (md) is defined in the first schedule of the health products act (hpa) and products that meet this definition are regulated. The imdrf guidance documents essential principles of safety and performance of. Diagnostic Medical Device Classification.
From www.thermofisher.com
IVDD vs. IVDR Classifications Defined and Compared OEMpowered Diagnostic Medical Device Classification Medical device (md) is defined in the first schedule of the health products act (hpa) and products that meet this definition are regulated. This document provides guidance to assist product owners in risk classification of in vitro diagnostic (ivd) medical devices using the. This guidance, relating to the application of regulation (eu) 2017/746 on in vitro diagnostic medical devices (ivdr). Diagnostic Medical Device Classification.
From mdrc-consulting.com
Downloads MDRC Diagnostic Medical Device Classification The imdrf guidance documents essential principles of safety and performance of medical devices and ivd medical devices and. The fda classifies medical devices, including ivd products, into class i, ii, or iii according to the level of regulatory control that is necessary to. This guidance, relating to the application of regulation (eu) 2017/746 on in vitro diagnostic medical devices (ivdr). Diagnostic Medical Device Classification.
From www.medicalmicromolding.com
UK Medical Device Classification Diagnostic Medical Device Classification Medical device (md) is defined in the first schedule of the health products act (hpa) and products that meet this definition are regulated. This document provides guidance to assist product owners in risk classification of in vitro diagnostic (ivd) medical devices using the. This scheme assigns diagnostic medical devices to one of four classes (a through d) based on ascending. Diagnostic Medical Device Classification.
From www.gilero.com
Medical Device Classification Overview of 3 Classes Gilero Diagnostic Medical Device Classification Medical device (md) is defined in the first schedule of the health products act (hpa) and products that meet this definition are regulated. This document provides guidance to assist product owners in risk classification of in vitro diagnostic (ivd) medical devices using the. Any active medical device, whether used alone or in combination with other medical devices, to. The imdrf. Diagnostic Medical Device Classification.
From www.scilife.io
In Vitro Diagnostics (IVD) A Complete Overview Scilife Diagnostic Medical Device Classification The fda classifies medical devices, including ivd products, into class i, ii, or iii according to the level of regulatory control that is necessary to. This document provides guidance to assist product owners in risk classification of in vitro diagnostic (ivd) medical devices using the. Any active medical device, whether used alone or in combination with other medical devices, to.. Diagnostic Medical Device Classification.
From www.presentationeze.com
FDA medical device classification PresentationEZE Diagnostic Medical Device Classification Medical device (md) is defined in the first schedule of the health products act (hpa) and products that meet this definition are regulated. Active device intended for diagnosis: Any active medical device, whether used alone or in combination with other medical devices, to. The fda classifies medical devices, including ivd products, into class i, ii, or iii according to the. Diagnostic Medical Device Classification.
From laegemiddelstyrelsen.dk
Classification of in vitro diagnostic medical devices (IVD) Diagnostic Medical Device Classification Medical device (md) is defined in the first schedule of the health products act (hpa) and products that meet this definition are regulated. The fda classifies medical devices, including ivd products, into class i, ii, or iii according to the level of regulatory control that is necessary to. This scheme assigns diagnostic medical devices to one of four classes (a. Diagnostic Medical Device Classification.
From www.gilero.com
Medical Device Classification Overview of 3 Classes Gilero Diagnostic Medical Device Classification The imdrf guidance documents essential principles of safety and performance of medical devices and ivd medical devices and. Any active medical device, whether used alone or in combination with other medical devices, to. This scheme assigns diagnostic medical devices to one of four classes (a through d) based on ascending risk to patients if the device malfunctions, using seven. Active. Diagnostic Medical Device Classification.